BIOTRONIK

|
2010 I worked for half a year at BIOTRONIK where I wrote my Diploma thesis entitled "Replication of Polyurethan
degradation through the influence of metal-ion-oxidation".
Biotronik is one of the world’s leading manufacturers of cardio- and endovascular medical devices.
It is headquartered in Berlin, Germany, and represented in over 100 countries by a global workforce
of more than 5600 employees. Several million patients have received BIOTRONIK implants designed to save
lives and improve quality of life. For more information about the company visit their website:
BIOTRONIK
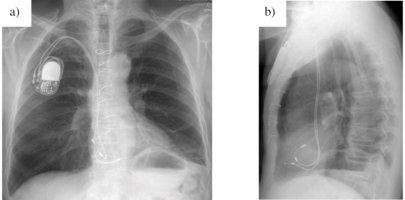
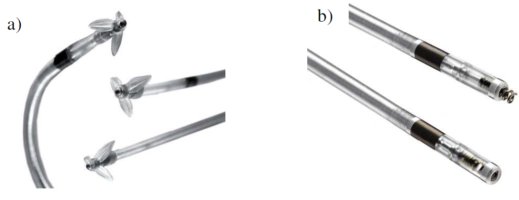
The main aim of my work was the investigation of new pacemaker electrode insulations and the quarantee of
proper function during the load of 400 million cardiac contractions (approximately 10 years of implantation).
Due to the code of silence and industrial secret no further details about the materials and results can be given.
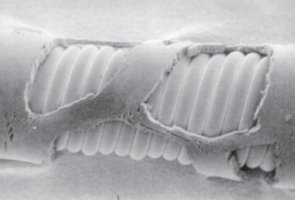
During this study the material was strained and aged chemically as well mechanically to iminate the real load of
cardiac contractions in vitro. Loaded samples were afterwards evaluated by several analysing methods, techniques and
evaluation tools:
- IR-Spectroscopy
- Differential scanning calorimetry (DSC)
- Thermogravimetric analysis (TGA)
- Tensile test
- Gel permeation chromatography (GPC)
- Atomic force microscopy
- Scanning electron microscopy
- Hardness testing
- Design of Experiment (DoE)